Moving from Paper Registers to 21 CFR Part 11 Compliant Electronic Drug Logs
Moving from Paper Registers to 21 CFR Part 11 Compliant Electronic Drug Logs The management process for Controlled substance tracking requires precise documentation according to established rules. The use of traditional paper registers results in three main problems: they require excessive time to complete, they contain errors, and their handling becomes difficult during audit processes. The implementation of an Electronic […]
How to Integrate ISO 13485 (Devices) and GMP (Pharma) for Combination Products
The growing need for drug-device combination products results in unique challenges which need to be solved through regulatory systems. The FDA needs companies to demonstrate their ISO 13485 and GMP compliance through the upcoming 2026 implementation of ISO 13485 for Quality Management System Regulation (QMSR 2026). Drug-device combination manufacturers need to understand the relationship between ISO 13485 and GMP standards to sustain […]
How to Handle “Hot Spots” and Humidity Spikes in 2026 Climate Extremes
The pharmaceutical and life sciences industries in the UK face mounting difficulties because of climate change, together with specific challenges that affect pharmaceutical logistics operations during extreme temperature and humidity variations, which will occur in 2026. The storage conditions in warehouses must meet compliance standards because they determine product quality, shelf life, and GDP storage requirements. The primary method for protecting […]
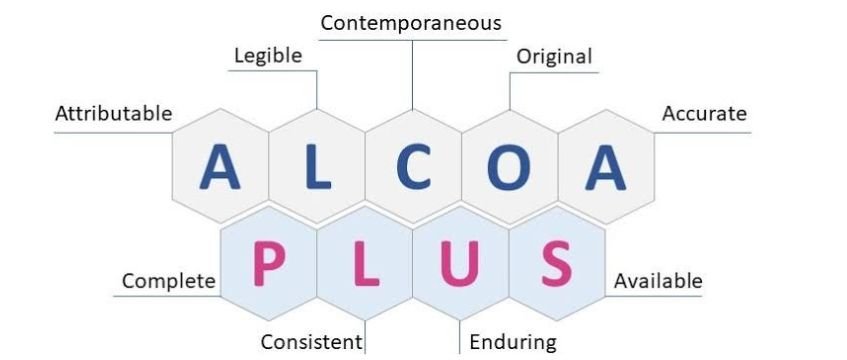
Why “ALCOA+” is the #1 Focus for Inspectors This Year
Why “ALCOA+” is the #1 Focus for Inspectors This Year Data integrity has become the primary focus of regulatory bodies in 2026, while inspectors use ALCOA+ principles as their primary evaluation standard for pharmaceutical and life sciences operations. The FDA and MHRA require organizations to maintain complete and accurate records, which makes ALCOA+ compliance mandatory […]
Roadmap to Compliance with the New Veterinary GMP (2025/2091)
The European Union’s Veterinary Good Manufacturing Practice (GMP) framework is undergoing development. The European Union regulation, which establishes requirements for all Marketing Authorisation Holders and manufacturers, will become effective on July 16 2026, according to the veterinary medicinal product regulations defined in Regulation EU 2019/6. UK businesses must understand this roadmap to prepare for their […]
Integrating QMS and GDP: End-to-End Quality Oversight Across the Supply Chain
The present-day world of pharmacy compels a strict monitoring of both production and distribution processes in order to ensure that the quality of a product is maintained throughout its life cycle. The merger of QMS and GDP not only ensures the quality of the entire supply chain but also improves the traceability and GxP compliance. The whole operation becomes unified and is no longer broken down into different […]
Omnichannel in Pharma: What True Digital Transformation Looks Like
The pharmaceutical sector is changing very fast due to the adoption of digital technology and the latter’s impact on the ways people interact with healthcare providers. Omnichannel engagement has become a basic strategy rather than a mere marketing trend nowadays, and thus it is the very basis of personalised, compliant and data-driven relationships of pharmaceutical companies with healthcare professionals (HCPs) and patients. The […]
Continuous Manufacturing: The Competitive Advantage Pharma Can’t Ignore
Continuous Manufacturing: The Competitive Advantage Pharma Can’t Ignore In a sector where precision, safety, and speed are paramount, continuous manufacturing has proven to be a groundbreaking technology for the pharmaceutical industry. The new approach allows processing of materials with no interruptions and at a real-time pace, which is not possible with the traditional batch systems. The product of this […]
Sustainability Meets Security: Green Supply Chains for a Changing Regulatory Climate
The evolving pharmaceutical landscape of today has made sustainability and regulatory compliance inseparable priorities. The transformation of pharmaceutical practices into sustainable ones is not only about reducing the carbon footprint but also about developing supply chain resilience and meeting the ESG (Environmental, Social, and Governance) commitments that regulators and investors increasingly expect. 1. How can pharma companies align sustainability and compliance? Pharmaceutical companies can simultaneously […]
Top FDA 483 Observations — and How to Avoid Them
Keeping a constant state of inspection readiness is the very foundation of GMP compliance in the pharmaceutical sector. Issuing Form 483 is a way that the U.S. FDA and other regulatory authorities, like it, carry out inspections and note down the deviations from the acceptable manufacturing practices. Such findings may lead to severe outcomes if […]