Why "ALCOA+" is the #1 Focus for Inspectors This Year
Data integrity has become the primary focus of regulatory bodies in 2026, while inspectors use ALCOA+ principles as their primary evaluation standard for pharmaceutical and life sciences operations. The FDA and MHRA require organizations to maintain complete and accurate records, which makes ALCOA+ compliance mandatory for all UK organizations before their upcoming inspections and audits.
The "+" Expansion
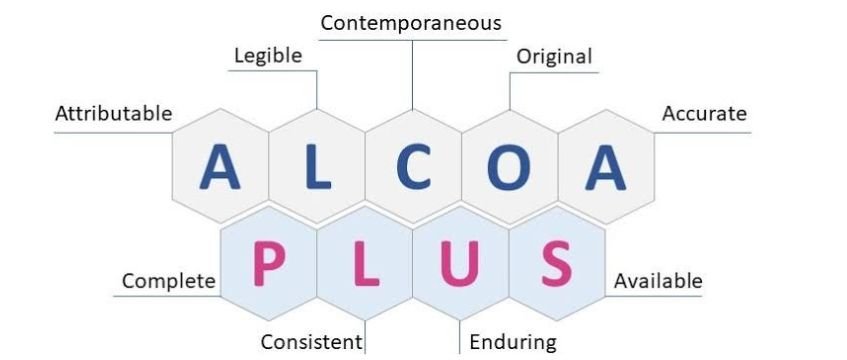
The original meaning of ALCOA was Attributable Legible Contemporaneous Original and Accurate. The “+ ” extension of these principles introduces Complete Consistent Enduring and Available principles to demonstrate how data management in regulated environments has become more intricate. Inspectors now expect organizations to ensure data is not only properly recorded but traceable, consistent, and accessible throughout its lifecycle. The current situation holds special importance for businesses that manage both digital and paper documentation.
Hybrid Records: Paper and Digital Risks
Many organizations continue to operate hybrid systems, which combine both paper and digital record-keeping methods. The systems create dangers through transcription errors, incomplete documentation, and version control problems. Inspectors focus on these hybrid workflows to ensure no data is lost or misrepresented during transfers between media types. The organization needs to confirm its digital and paper documents to ALCOA+ standards, which will provide proof of complete data integrity.
Audit Trail Review
Current inspection procedures use audit trails as their primary inspection method. The system records each data event with its respective time and date, which enables organizations to identify human mistakes and investigate potential fraudulent activities. The inspectors examine these logs to check whether all modifications have proper reasons and complete GxP documentation. The organization needs to maintain complete audit trails while conducting regular reviews to detect unusual activities, which is essential for ALCOA+ compliance.
Human Error vs. Fraud
Most deviations stem from human error, which includes missed entries, illegible handwriting and delayed documentation. The inspectors receive training to detect possible fraudulent activities. The distinction requires understanding because human error needs process improvements and staff training, whereas fraud requires regulatory enforcement. The organization can decrease risks through its two strategies of proactive monitoring and regular staff education.
Conclusion
Organizations demonstrate their dedication to trustworthy data through their implementation of ALCOA+ principles. UK companies use the standards to prepare for FDA and MHRA inspections while establishing responsibility through their record-keeping systems. The organization protects its data integrity by addressing the “plus” components through active measures.
FAQs
- Is ALCOA+ a legal requirement?
The FDA guidelines, the MHRA guidelines and the EU GxP guidelines all make references to it, although it does not serve as a primary source. Compliance is effectively mandatory to demonstrate data integrity during inspections. - What is the most common ALCOA+ failure?
The most common issues are incomplete or inconsistent records, especially in hybrid or legacy systems. - How do we address data integrity in legacy systems without audit trails?You need to establish compensating controls, which involve implementing secondary verification, creating manual logs and establishing procedural oversight during your system upgrade process. The measures need to be documented because they require documentation