Trusted Expertise in Pharmaceutical Quality System (PQS) and Quality Management
At Pharma Quality Service Ltd., quality systems and services are maintained and in compliance with Pharmaceutical Quality System standards in organisations all over the UK. In contrast to an environment where quality and compliance can mean the company paying costs for delays or achieving operational excellence, our services help achieve operational excellence in pharmaceutical, biotechnology, and medical-device interventions.
The life sciences sector is ranked as one of the fastest-growing industries in the UK, developing healthcare innovations and the state’s economy on either side of the spectrum. But opportunity is not without responsibility. Organizations should ensure that every process operates strictly concerning GMP/GMDP and other MHRA requirements. Thus PQSL is that assurance partner to keep compliant and to keep your innovation free from compromise.
What is a Pharmaceutical Quality System (PQS)?
Pharmaceutical Quality Systems (PQS) is the framework for the organisation, science, and risk-based approach that supports all actions taken in pharmaceutical operations. The PQS concept inculcates a quality culture beginning from raw material sourcing until product distribution. It is designed to protect the patient, protect the company’s reputation, and secure regulatory approval.
In the UK, the MHRA acts as a very stringent overseer of pharmaceutical and biotech companies; hence, the establishment of an efficient PQS is mandatory. It would also help companies prepare for inspection so as to minimise any risk of infractions and ensure conformity with standards in both UK and EU GMP. In addition to pharmaceuticals, PQS also applies to biotechnology companies engaged in the development of advanced therapy medicines and medical device companies seeking conformity in alignment with MDR and UKCA.
If quality becomes part of your operations through PQS, then it becomes much more than just compliance with standards but surely an opportunity for competitive advantage.
Our Courses
- Introduction to GMDP Regulations and Importance of GMDP
- Pharmaceutical Quality System (PQS)
- Personnel
- Premises and Equipments
- Documentation
- Production
- Quality Control
- Outsource Activities
- Complaints, Quality Defects, and Product Recalls
- Self-Inspection and Audit
- Good Distribution Practice (cGDP)
- Regulatory Affairs & Regulatory Submissions
Pharmaceutical Quality Management System Explained
While PQS forms the foundation, the Pharmaceutical QMS forms the broader frame encompassing the processes, people, and documentation. It can guarantee consistent functionality of all processes through research and development all the way through commercial manufacture.
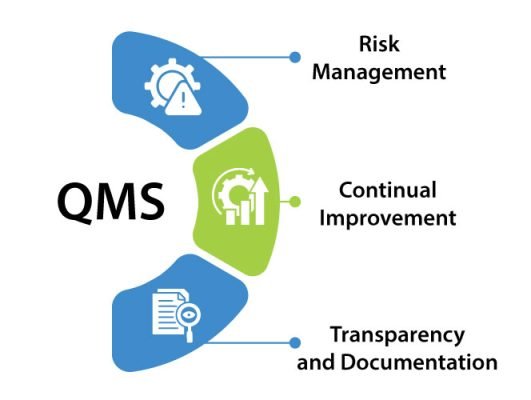
An efficient Pharmaceutical QMS focuses on:
- Risk Management – specially the identification and mitigation of potential risks of quality or compliance.
- Continual Improvement – keeping systems that evolve in reference to regulatory updates and market changes.
- Transparency and Documentation – refers to having a clear set of records that suffice for regulatory agencies such as MHRA.
For UK-based companies, a robust QMS ensures the seamless conduct of audits, efficacy of MHRA inspections, and timely approvals of products. It remains incumbent for the teams to stay inspection-ready at all times, while bringing about cultural change towards proactive quality management.
Why PQSL Matters for UK Clients
We understand, at PQSL, that each organisation suffers its own peculiar challenges. That is why we never go for a one-size-fits-all strategy, but, quite the contrary, we customise our services for the clients in the pharmaceutical and biotech sectors in the UK.
Services we offer are:
- Specialised Training Programmes–for empowering Quality Teams with practical knowledge and best practices in industry.
- Team of Consultants-Create, implement and optimize PQS and QMS frameworks to meet the legal requirements.
- Regulatory Support–Generally for assisting companies in preparing for MHRA inspections, UKCA requirements, and inspections by global regulators.

The Advantages of Working with PQSL in the United Kingdom
When PQSL is chosen as a quality partner, clients benefit from:
- Streamlining processes ensures a higher degree of efficiency.
- This leads to a reduced chance of regulatory fines and product recalls.
- Expert advisories are offered in line with GMP, GMDP, MDR, or UKCA.
- Tailored solutions relevant to both mature business and emerging startups.
- Greater audit preparedness and lesser stress.
Our mission is to assist you in transforming compliance into an enabler rather than a burden.
Industries & Applications
PQSL supports clients of pharmaceutical industry:
- Pharmaceutical Industries – Ensures compliance for drug manufacturing , Research and Development from clinical trials to scale manufacture.
- Biotechnology – Supports companies that develop innovative therapies, encompassing cell and gene treatment and cutting-edge biologics.
- Medical Devices- Provided compliance assistance: MDR and UKCA and assistance to device manufacturers for approvals and maintenance of the market.
By partnering with PQSL, your organisation can concentrate on innovation and patient outcomes, while we deal with the nitty-gritties of compliance.
Partner with PQSL Today
With such a competitive business atmosphere prevailing today in the UK, pharmaceutical, biotechnology, and medical-device companies cannot abuse compliance. PQSL has the expertise, tools, and training for building strong Pharmaceutical Quality Systems (PQS) and efficient Pharmaceutical Quality Management Systems that keep you inspection-ready and future-proof.
Contact Pharma Quality Services Limited today to find out how we can support your compliance journey and strengthen the quality framework of your organisation.





